Denoising scRNAseq#
In this tutorial, we will walk through the steps for denoising mRNA counts in species-mixing data.
In this experiment, an equal number of human HEK293T cells and mouse NIH3T3 cells were pooled and sequenced, transcripts from the other organism were unambiguously ambient contamination. The original dataset is downloaded from 10x genomics dataset, and cell annotation file is available at scAR-reproducibility/data
[ ]:
# Run this cell to install scar in Colab
# Skip this cell if running on your own device
%pip install scanpy
%pip install git+https://github.com/Novartis/scAR.git
%pip install matplotlib==3.1.3 # Specify this matplotlib version to avoid errors
[1]:
import numpy as np
import pandas as pd
import matplotlib.pyplot as plt
import seaborn as sns
import scanpy as sc
from scar import model, setup_anndata
import warnings
warnings.simplefilter("ignore")
Download data#
The raw and filtered count matrices can be downloaded from 10x Dataset.
The raw data#
cellranger output: raw_feature_bc_matrix
[2]:
hgmm_20k_raw = sc.read_10x_h5(filename='20k_hgmm_3p_HT_nextgem_Chromium_X_raw_feature_bc_matrix.h5ad',
backup_url='https://cf.10xgenomics.com/samples/cell-exp/6.1.0/20k_hgmm_3p_HT_nextgem_Chromium_X/20k_hgmm_3p_HT_nextgem_Chromium_X_raw_feature_bc_matrix.h5');
hgmm_20k_raw.var_names_make_unique();
The filtered data#
cellranger output: filtered_feature_bc_matrix
[3]:
hgmm_20k = sc.read_10x_h5(filename='20k_hgmm_3p_HT_nextgem_Chromium_X_filtered_feature_bc_matrix.h5ad',
backup_url='https://cf.10xgenomics.com/samples/cell-exp/6.1.0/20k_hgmm_3p_HT_nextgem_Chromium_X/20k_hgmm_3p_HT_nextgem_Chromium_X_filtered_feature_bc_matrix.h5');
hgmm_20k.var_names_make_unique();
Annotation and filtering#
We annotated cells by their cell species and provided the annotation file
[4]:
!git clone https://github.com/CaibinSh/scAR-reproducibility.git
hgmm_20k_anno = pd.read_csv('scAR-reproducibility/data/20k_hgmm_cell_annotation.csv', index_col=0)
hgmm_20k.obs=hgmm_20k.obs.join(hgmm_20k_anno[['species']])
fatal: destination path 'scAR-reproducibility' already exists and is not an empty directory.
Gene and cell filtering
[5]:
sc.pp.filter_genes(hgmm_20k, min_counts=200);
sc.pp.filter_genes(hgmm_20k, max_counts=6000);
sc.pp.filter_cells(hgmm_20k, min_genes=200);
Calculate number of human and mouse transcripts
[6]:
hgmm_20k.obs['human gene counts'] = hgmm_20k[:, hgmm_20k.var['genome']=="GRCh38"].X.sum(axis=1).A1
hgmm_20k.obs['mouse gene counts'] = hgmm_20k[:, hgmm_20k.var['genome']=="mm10"].X.sum(axis=1).A1
[7]:
hgmm_20k
[7]:
AnnData object with n_obs × n_vars = 16292 × 16586
obs: 'species', 'n_genes', 'human gene counts', 'mouse gene counts'
var: 'gene_ids', 'feature_types', 'genome', 'n_counts'
Estimate ambient profile#
See details in calculating ambient profile
[8]:
setup_anndata(
adata = hgmm_20k,
raw_adata = hgmm_20k_raw,
prob = 0.995,
kneeplot = True
)
2023-05-01 18:40:23|INFO|setup_anndata|Randomly sample 199666 droplets from 199666 droplets.
2023-05-01 18:40:23|INFO|setup_anndata|Estimating ambient profile for ['Gene Expression']...
2023-05-01 18:40:49|INFO|setup_anndata|Iteration: 1
2023-05-01 18:41:13|INFO|setup_anndata|Iteration: 2
2023-05-01 18:41:38|INFO|setup_anndata|Iteration: 3
2023-05-01 18:41:38|INFO|setup_anndata|Estimated ambient profile for Gene Expression saved in adata.uns
2023-05-01 18:41:38|INFO|setup_anndata|Estimated ambient profile for all features saved in adata.uns

[9]:
hgmm_20k.uns["ambient_profile_Gene Expression"].head()
[9]:
| ambient_profile_Gene Expression | |
|---|---|
| GRCh38_AL627309.5 | 0.000000 |
| GRCh38_LINC01128 | 0.000127 |
| GRCh38_LINC00115 | 0.000000 |
| GRCh38_FAM41C | 0.000010 |
| GRCh38_AL645608.2 | 0.000010 |
Training#
Note
However, in the case of data with only highly variable genes, a smaller sparsity parameter is recommended to avoid overcorrection.
[10]:
hgmm_20k_scar = model(raw_count=hgmm_20k, # In the case of Anndata object, scar will automatically use the estimated ambient_profile present in adata.uns.
ambient_profile=hgmm_20k.uns['ambient_profile_Gene Expression'],
feature_type='mRNA',
sparsity=1,
device='cuda' # Both cpu and cuda are supported.
)
hgmm_20k_scar.train(epochs=200,
batch_size=64,
verbose=True
)
# After training, we can infer the native true signal
hgmm_20k_scar.inference(batch_size=256) # by defaut, batch_size = None, set a batch_size if getting a memory issue
2023-05-01 18:41:40|INFO|model|cuda will be used.
2023-05-01 18:41:47|INFO|VAE|Running VAE using the following param set:
2023-05-01 18:41:47|INFO|VAE|...denoised count type: mRNA
2023-05-01 18:41:47|INFO|VAE|...count model: binomial
2023-05-01 18:41:47|INFO|VAE|...num_input_feature: 16586
2023-05-01 18:41:47|INFO|VAE|...NN_layer1: 150
2023-05-01 18:41:47|INFO|VAE|...NN_layer2: 100
2023-05-01 18:41:47|INFO|VAE|...latent_space: 15
2023-05-01 18:41:47|INFO|VAE|...dropout_prob: 0.00
2023-05-01 18:41:47|INFO|VAE|...expected data sparsity: 1.00
2023-05-01 18:41:47|INFO|model|kld_weight: 1.00e-05
2023-05-01 18:41:47|INFO|model|learning rate: 1.00e-03
2023-05-01 18:41:47|INFO|model|lr_step_size: 5
2023-05-01 18:41:47|INFO|model|lr_gamma: 0.97
Training: 100%|██████████| 200/200 [08:12<00:00, 2.46s/it, Loss=4.7369e+03]
The denoised counts are saved in hgmm_20k_scar.native_counts and can be used for downstream analysis.
[11]:
denoised_count = pd.DataFrame(hgmm_20k_scar.native_counts, index=hgmm_20k.obs_names, columns=hgmm_20k.var_names)
denoised_count.head()
[11]:
| GRCh38_AL627309.5 | GRCh38_LINC01128 | GRCh38_LINC00115 | GRCh38_FAM41C | GRCh38_AL645608.2 | GRCh38_LINC02593 | GRCh38_SAMD11 | GRCh38_KLHL17 | GRCh38_AL645608.7 | GRCh38_ISG15 | ... | mm10___Gm21887 | mm10___Kdm5d | mm10___Eif2s3y | mm10___Uty | mm10___Ddx3y | mm10___AC133103.1 | mm10___AC168977.1 | mm10___CAAA01118383.1 | mm10___Spry3 | mm10___Tmlhe | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AAACCCAAGAGCCGAT-1 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 1.0 | ... | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| AAACCCAAGCGTTCCG-1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 0.0 | 0.0 | 1.0 | ... | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| AAACCCAAGTGCGTCC-1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ... | 0.0 | 1.0 | 1.0 | 0.0 | 2.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| AAACCCACAAACTGCT-1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ... | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| AAACCCACAATACCTG-1 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | ... | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
5 rows × 16586 columns
Visualization#
Calculate number of human and mouse transcripts before denoising
[12]:
raw_counts_df = hgmm_20k.obs[['human gene counts', 'mouse gene counts']].astype(int)
raw_counts_df['log2(human gene counts+1)'] = np.log2(raw_counts_df['human gene counts']+1)
raw_counts_df['log2(mouse gene counts+1)'] = np.log2(raw_counts_df['mouse gene counts']+1)
raw_counts_df = raw_counts_df.join(hgmm_20k.obs[['species']])
visualization of raw counts
[13]:
plt.figure(figsize=(4, 4), dpi=150)
ax = sns.jointplot(data=raw_counts_df,
x="log2(human gene counts+1)",
y="log2(mouse gene counts+1)",
hue='species',
hue_order=['HEK293T', 'mixture', 'NIH3T3'],
s=8,
alpha=0.5,
legend=True,
height=5,
marginal_kws={'common_norm':False})
ax.ax_joint.text(0, 0, 'raw counts')
ax.ax_joint.set_xlim(-1, 14)
ax.ax_joint.set_ylim(-1, 14)
ax.ax_joint.legend(bbox_to_anchor=(1.55, 0.6), borderaxespad=0);
<Figure size 600x600 with 0 Axes>

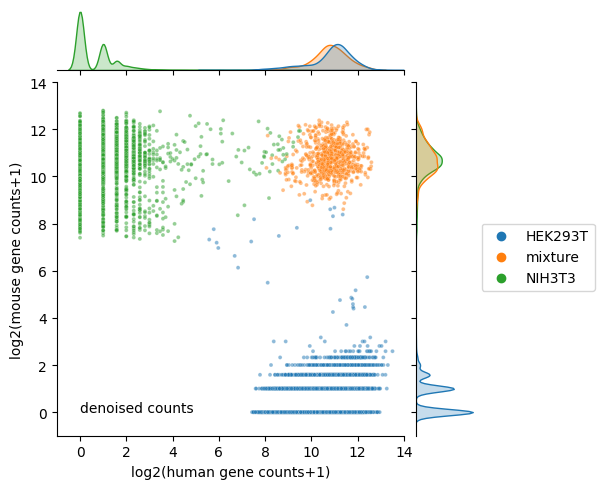
Calculate number of human and mouse transcripts after denoising
[14]:
denoised_count['human gene counts'] = denoised_count.loc[:,denoised_count.columns.str.startswith('GRCh38_')].sum(axis=1)
denoised_count['mouse gene counts'] = denoised_count.loc[:,denoised_count.columns.str.startswith('mm10_')].sum(axis=1)
denoised_count['species'] = raw_counts_df['species']
denoised_count['log2(human gene counts+1)'] = np.log2(denoised_count['human gene counts']+1)
denoised_count['log2(mouse gene counts+1)'] = np.log2(denoised_count['mouse gene counts']+1)
visualization of denoised counts
[15]:
plt.figure(figsize=(4, 4), dpi=150);
ax = sns.jointplot(data=denoised_count,
x="log2(human gene counts+1)",
y="log2(mouse gene counts+1)",
hue='species',
hue_order=['HEK293T', 'mixture', 'NIH3T3'],
s=8,
alpha=0.5,
legend=True,
height=5,
marginal_kws={'common_norm':False});
ax.ax_joint.text(0, 0, 'denoised counts')
ax.ax_joint.set_xlim(-1, 14)
ax.ax_joint.set_ylim(-1, 14)
ax.ax_joint.legend(bbox_to_anchor=(1.55, 0.6), borderaxespad=0);
<Figure size 600x600 with 0 Axes>